MAESTRO integrates CTMS, eTMF, QMS, and intelligent workflows into a unified platform, eliminating operational silos while leveraging AI to automate routine tasks, ensure regulatory compliance, and drive unprecedented research efficiency.
MAESTRO transforms fragmented research operations into a cohesive, intelligent ecosystem by eliminating data silos and connecting every aspect of your clinical trials.
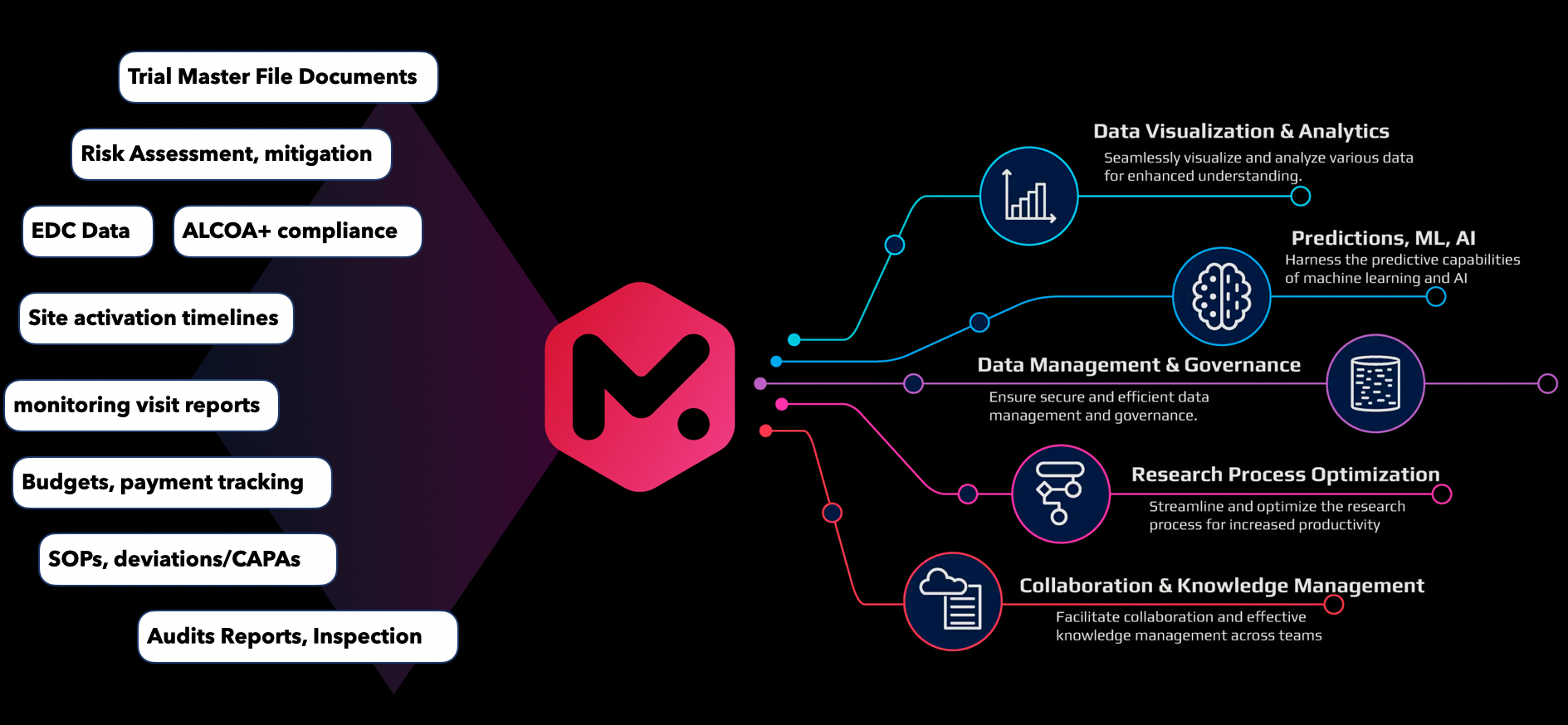
MAESTRO seamlessly integrates with EDC systems, risk assessments, monitoring reports, SOPs, and audit documentation, creating a single source of truth that eliminates conflicting information and duplicative work.
Our AI-powered platform automatically processes and contextualizes information from multiple sources, delivering real-time insights and predictive analytics that drive better decision-making across your organization.
Break down barriers between teams with unified workflows that enable seamless collaboration, ensuring everyone has immediate access to the information they need while maintaining strict compliance controls.
MAESTRO directly addresses the key obstacles hindering research progress and operational efficiency in life sciences.
Eliminate disconnected systems with a unified platform that ensures seamless data flow between modules, creating a single source of truth that enhances collaboration and visibility across your research portfolio.
Automate documentation processes with intelligent document processing using AI to generate, classify, and validate documentation, reducing manual effort by up to 60% while improving accuracy.
Ensure 99.8% compliance with automated checks against FDA, EMA, and ICH guidelines, identifying potential issues before they become problems with real-time regulatory updates.
Streamline planning, execution, and monitoring with comprehensive tools for every stage of the trial lifecycle, enhanced by predictive analytics to prevent bottlenecks.
Implement consistent quality processes with real-time monitoring that automates quality event detection, root cause analysis, and CAPA implementation for GxP compliance.
Transform fragmented data into actionable insights with AI-powered analytics that uncover patterns, predict outcomes, and identify optimization opportunities through customizable dashboards.
MAESTRO combines industry-leading modules with AI-powered workflows.
Streamlined management of all clinical trial aspects.
AI-powered trial documentation management.
Comprehensive quality control and compliance.
Streamlined operations with smart process automation.
Autonomous agents for complex task automation.
Automated processing and templates slash documentation overhead.
Streamlined workflows accelerate trial initiation processes.
AI-powered validation ensures near-perfect compliance.
Automation of routine tasks focuses teams on innovation.
Unify all research operations in a single platform.
Continuous monitoring and analytics for better decisions.
Our platform integrates cutting-edge AI with robust data management for a comprehensive solution that adapts to your needs.

Advanced AI engine that processes research data, automates tasks, and provides predictive insights. Includes specialized models for document processing, NLP, anomaly detection, and analytics that continuously learn while maintaining compliance.
Centralized repository that eliminates silos and enables cross-functional analytics. Maps complex relationships between trials, documents, quality events, and metrics to create a complete digital representation of research activities.
Connect with existing systems through our API framework, supporting both legacy and modern technologies. Includes pre-built connectors, flexible REST API, and specialized adapters for clinical data standards with comprehensive audit trails.
MAESTRO's flexible architecture deploys seamlessly across all major cloud providers, giving you freedom of choice without vendor lock-in.
Choose the cloud provider that best meets your regulatory, cost, and geographic requirements.
Maintain compliance with regional data regulations by selecting appropriate cloud regions.
Seamlessly operate across public cloud, private cloud, and on-premises environments.
For large pharmaceutical companies
For universities and research centers
For biotech and early-stage companies
Contact our team to design a tailored implementation that meets your specific research requirements.
Contact for Custom SolutionMAESTRO was created by RAN BIOLINKS CANADA, a company founded by scientists with extensive clinical and research experience who recognized a critical problem in the industry: fragmented tools creating artificial data silos.
While numerous solutions exist in the market, we observed that their separation is primarily driven by business models rather than scientific needs. This fragmentation forces researchers to make difficult choices—either invest in multiple disconnected systems or sacrifice essential capabilities due to budget constraints.
Our philosophy is fundamentally different. We believe clinical scientists deserve a unified ecosystem where all tools work seamlessly together, allowing them to focus on breakthrough discoveries rather than managing disjointed software. MAESTRO embodies this vision, combining traditionally separate systems into one cohesive platform that puts scientists first.

See how MAESTRO can transform your clinical operations. Schedule a personalized demo with our team to explore the platform's capabilities and discuss your specific needs.
Join organizations achieving faster study startups, real-time TMF inspection readiness, and automated site management—all while saving hours daily per team member.
Book Your Discovery Call